Day 1 :
Keynote Forum
Nicolas Abatzoglou
Université de Sherbrooke, Canada
Keynote: Techno-economic data and feasibility of hydrogen production from biogas using a novel low cost Ni-based reforming catalyst derived from metallurgical residues
Time : 10:30-11:10

Biography:
Abstract:
Keynote Forum
Ignacio Gracia
University of Castilla-La Mancha, Spain
Keynote: Crossing the gap between research and market in chemical engineering: Application to supercritical technology
Time : 11:30-12:10

Biography:
Abstract:
Keynote Forum
Davis L. Ford
The University of Texas at Austin, USA
Keynote: The past and future of enhanced oil and gas extraction in the United States
Time : 12:10-12:50

Biography:
Abstract:
- Track 1: Chemical Engineering
Track 2: Electrochemistry and Electrochemical Engineering
Track 6: Biochemical Engineering
Location: ZURICH

Chair
Ignacio Gracia
University of Castilla-La Mancha, Spain

Co-Chair
Jong Moon Park
Pohang University of Science and Technology, Korea
Session Introduction
Anil Oroskar
Orochem Technologies Inc., USA
Title: Opportunities for innovation in chemical industry
Time : 12:50-13:20

Biography:
Anil Oroskar is a Founder and Chief technology Officer at Orochem Technologies Inc. USA. Also he is an Adjunct Professor of Chemical Engineering at University of Illinois, Chicago. He has more than 35 years of experience in the field of Designed refinery processes , refinery & petrochemical process improvements, engineering, technical service, operations management. He received his PhD in ChemE at University of Wisconsin, Madison in 1981. He has more than 50 US Patents and Key participant over 16 International conferences. He was one of the Directors of AICHE Fuels and Petroleum Division and has focused recently on the development of Biotechnology, New Energy Technologies, Fuel Cells Technology and Micro-Reaction Technology.
Abstract:
Real growth of chemical industry started in the early 1900s after the discovery and growth of crude oil. The switch from coal to oil spurred innovations in fuels as well as petrochemicals. There was rapid growth in innovative processes and products which improved quality of life for all people on earth. Unfortunately by the end of last century this innovation had slowed down. By the year 2000 Chemical Industry had become a mature “brick and mortar” industry with very few breakthroughs in processes and products. Focus in the last two decades has primarily been in improving information and knowledge leading to more automated chemical processes.
Fortunately there still are many opportunities for significant innovations in chemical industry. These opportunities exist because there are significant needs presented by the world we live in. These opportunities can be classified in:
1. Improved Resource Utilization such as drinking water from seawater, olefins from Natural gas etc.
2. Improved Process efficiency such as Improved catalysts for petroleum cracking, improved electrochemical process for aluminum etc.
3. Reduced environmental impact such as reduced CO2 emissions, CO2 sequestration
4. Alternative feed stocks such as Cellulosic ethanol
This presentation will provide a summary of the growth and slowing down of chemical industry innovations during the last century and will highlight specific opportunities for spurring innovation in products and processes which could play an important part in renewal of chemical industry during this century.Real growth of chemical industry started in the early 1900s after the discovery and growth of crude oil. The switch from coal to oil spurred innovations in fuels as well as petrochemicals. There was rapid growth in innovative processes and products which improved quality of life for all people on earth. Unfortunately by the end of last century this innovation had slowed down. By the year 2000 Chemical Industry had become a mature “brick and mortar” industry with very few breakthroughs in processes and products. Focus in the last two decades has primarily been in improving information and knowledge leading to more automated chemical processes.
Said Al-Hallaj
University of Illinois, Chicago, USA
Title: Preventing thermal runaway propagation in li-ion batteries
Time : 14:10-14:40

Biography:
Abstract:
Luca Gael Bertoluzzi
Stanford University, USA
Title: Identifying electrochemical limitations of solar energy storage
Time : 14:40-15:10

Biography:
Abstract:
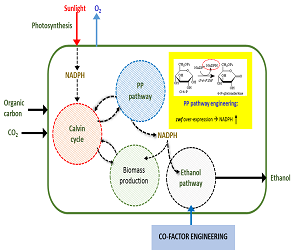
Jong Moon Park
Pohang University of Science and Technology, South Korea
Title: Enhancement of cyanobacterial ethanol production by co-factor engineering
Time : 15:10-15:40

Biography:
Abstract:
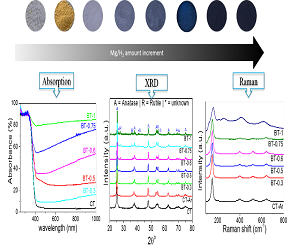
Jong-Sung Yu
Daegu Gyeongbuk Institute of Sicence & Technology, South Korea
Title: Highly effi cient oxygen-defi cient reduced Tio2-x for sunlight-induced water splitting for H2 generation
Time : 16:00-16:30

Biography:
Abstract:
Lara Fernandez-Cerezo
University College London, UK
Title: An ultra scale-down method to predict diafi ltration performance during formulation of concentrated mAb solutions
Time : 16:30-16:50

Biography:
Abstract:
Day 1 Program Closed by 17:00
Anderson José Beber
Solenis Water Technologies, Brazil
Title: Reduction on Water Consumption on a Cooling Tower With The Application of a Novel Biocide

Biography:
Anderson José Beber has over 17 year experience in industrial water treatment, especially clarification, demineralization, reverse osmosis, low and high pressure boiler water treatment and cooling water treatment. He has worked for different multinational water treatment companies, servicing serveral industries: pulp and paper, power, steel, manufacturing, food and beverage, automotive and many others. Over the past 6 years Anderson Beber has dedicated his expertise on special projects and technical assistance to Solenis sales team, being responsible for new product launch, technical training, project development, consultancy for boiler and cooling water treatment for industries in Latin America.
Abstract:
Microbiological control is essential in any cooling water system. A cooling system such as a large cooling tower is an excellent environment for micrboloigcal growth: water,
warm temperature, oxygen, dust and debries from air,
nutrients and others are some of the variables that contribute largely to the growth of microbiology colonies. The main negative consequence is that the biofilm (sludge) formed is highly insulating. It is known that biofilm is more insulating that CaCO3 or SiO2 scales. The best and less expensive way to control MB is by using large amounts of oxidizing biocides like chlorine gas, hypochlorite, bromine, chlorine dioxide. The goal is to maintain an oxidizing environment which is not friendly for bio cells. However, strong oxidizer may cause high chloride content, lower concentration cycles, higher cost among others. Also, a high oxidant environment may lead to higher corrosion rates. And finally the strong oxidizers are not selective, reacting to any contamination not only MB.
This paper shows the results of the application of a novel mild oxidizer on a large cooling tower at a power plant. This specific cooling tower utilizes grey water (tertiary treated domestic sewage) as make up water. After the application of this mild oxidizer, the concentration cycles were enhanced from an average of 4 up to 6.5, resulting in large savings to the plant. Also, stainless steel corrosion rates droped significantly due to the reduction of chlorides and sulfates residuals.
