Jong-Soo Lee
Daegu Gyeongbuk Institute of Science and Technology, South Korea
Title: Hybrid metal-Cu2S nanostructures as effi cient co-catalysts for photocatalytic hydrogen generation
Biography
Biography: Jong-Soo Lee
Abstract
In recent years, hybrid nanocrystals (HNs) have emerged as an important class of materials to tune the optical, electrical, magnetic and catalytic properties of nanocrystals. In HNs, two disparate functional material systems (i.e., metal/magnet, metal/semiconductor and magnet/semiconductor) are combined through their crystal facets, which results in the nontrivial synergetic effects including extinction enhancement due to the coupling of surface plasmon resonance and electronic doping by the intraparticle charge transfer. Among diff erent types of HNs, metal-semiconductor HNs are of particular interest in photocatalysis because it can provide a very good light absorbing semiconductor properties and catalytically active metal nanostructure properties. In the previous report, Pt-CdS/Se NCs exhibited high photocatalytic activity as well as stability. However, the toxicity associated with cadmium based semiconductors has driven research into possible alternative materials. Copper (I) sulfi de (Cu2S), a p-type semiconductor with a narrow bandgap of 1.2 eV, has been explored as a light absorber in photovoltaics and optoelectronic devices due to its nontoxic and earth-abundant constituents.
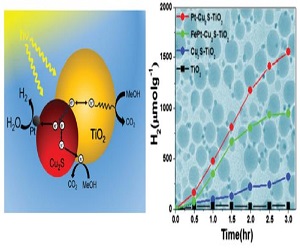
Also, Cu2S nanostructures have also shown high catalytic activity for polysulfi de redox systems in quantum-dot-sensitized solar cells. Cu2S nanocrystals with diverse shapes have been synthesized and employed in various applications. However, the synthesis and application of metal-Cu2S HNs have rarely been reported. In this presentation, we introduce a new synthesis method for the fabrication of hybrid metal-Cu2S (M=Pt, FePt) nanocrystals (HNs). Th e metal-Cu2S HNs were investigated in photocatalytic hydrogen generation as eff ective co-catalysts on TiO2. Th e Pt-Cu2S/TiO2 catalyst showed higher hydrogen generation rate compared with a pure TiO2 catalyst. This enhancement is attributed to the synergetic eff ects between the Cu2S and Pt, which signifi cantly improves the light absorption ability and the charge separation activity.