Kirsten Grubel
Ruhr-University Bochum, Germany
Title: Experimental solubility study on the high-pressure absorption of small molecules in ethanol oxidation reactions
Biography
Biography: Kirsten Grubel
Abstract
For commercial production of basic and fine chemicals, like aldehydes, ketones and dioles, alcohol oxidations are commonly used. Th ese processes suff er from the use of toxic oxidizing agents and the production of a vast amount of toxic by-products. In this regard, an important fi eld in chemical research is catalyst development in order to substitute commercial toxic substrates and to reduce environmental pollution. The heterogeneously catalyzed aerobic ethanol oxidation features the utilization of oxygen from air as oxidizing agent. This approach is not only cost and atom effi cient, it also bears the benefit of a greener industrial process since oxygen is non-toxic, readily available and water is the only by-product. Unfortunately, little is known about the solubility properties of the air components N2 and O2 in aqueous ethanol solutions at process conditions. This lack of data impedes to find optimal reaction conditions and to develop optimal catalyst with desired requirements. Within this project, solubility properties of the air components in dependence of temperature, pressure and liquid phase compositions are measured.
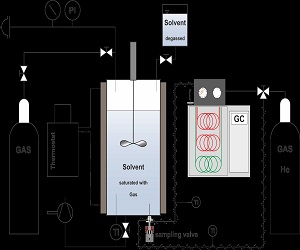
The setup for the gas solubility measurements mainly consists of a high pressure autoclave connected to a gas chromatograph. To investigate the solubility properties of a gas, the autoclave is filled with thoroughly degassed ethanol/water mixtures, heated to the desired temperature, pressurized with the gas to be analyzed and the mixture is stirred to accelerate equilibration times. At equilibrium conditions, samples from the liquid phase are withdrawn via the capillary of the sampling valve and preceded to the gas chromatograph via the superheated, helium purged transfer line, in order to evaporate the liquid sample content. By means of the at-line chromatograph, the molar amount of dissolved gases in the solvent (ethanol water mixtures) can be quantitatively determined. These data will help to optimize reaction conditions and to evaluate catalyst requirements for optimal efficiency and selectivity.